Synthetic diamonds, along with several other super-hard materials, such as cubic boron nitride used for cutting steel, have been manufactured for years. However, despite their usefulness, the complex and expensive production of these materials, which requires artificial generation of the ultra strong pressures under which natural diamonds are formed continues to be a major drawback.
UCLA scientists working under Professor Richard B. Kaner decided to try a different approach to creating super-hard materials. According to Kaner there are two ways to produce super-hard materials that are resistant to shape deformation or “ultra-incompressible”, a necessary condition for hardness. One way is to imitate natural diamond formation by combining carbon with boron or nitrogen so as to maintain short bonds. The second way is to find metals that are already incompressible and try to make them hard. Kaner and his team chose the second method and worked on making a naturally soft and incompressible metal harder.
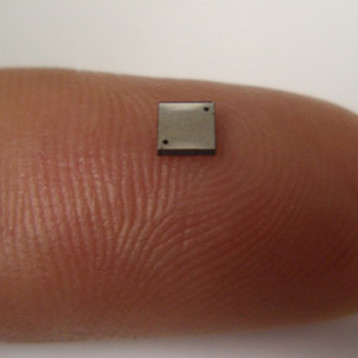
The team put a mixture of rhenium and boron powder in a furnace and ran a large electric current through it. This quickly melted the materials, allowing the formation of small ingots, just several centimeters long. The result is an ultra-hard material with a relatively low-energy production process, which is fairly inexpensive to produce in large quantities. Although the material is not quite as hard as a diamond, it is still very hard and can be used for a variety of applications.