|
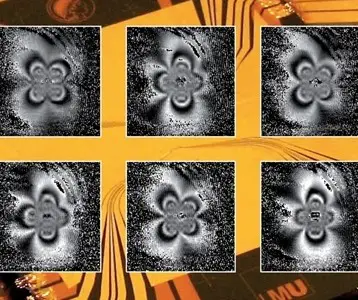
A quantum jump is a change of an electron from one quantum state to another within an atom. It appears to be discontinuous; the electron “jumps” from one energy level to another very quickly, after existing briefly in a state of superposition. The time this takes relates to the pressure broadening of spectral lines. Quantum jumps cause the emission of electromagnetic radiation, including that of light, which occurs in the form of quantized units called photons. Experimentally, these quantum jumps translate to discrete interruptions of the continuous emission from single molecules, revealing a phenomenon known as fluorescent intermittency or “blinking.”
Known physicist Neils Bohr, a Noble Prize-winner, predicted more than a century ago a phenomenon called “quantum jumps.” Although controversial in Bohr’s time, such quantum jumps were observed experimentally, and his prediction verified in the 1980s. During the 1990s, the technology to image single molecules matured, and it became possible to observe similar jumps in individual molecules.
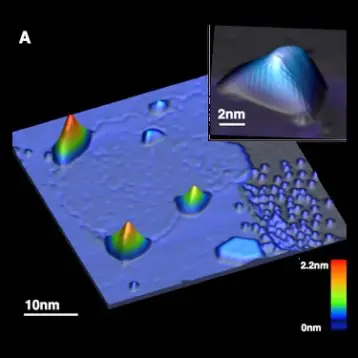
Apparently, Bohr’s theory is incomplete, as his predictions are fulfilled only in some cases. For many years scientists tried to understand what the cause was behind this inconsistency; to do so they tried to isolate the cases in which the observed fluorescence intermittency did not follow Bohr’s predictions. Specifically, in systems including individual inorganic nanostructures, clear deviations occur.
In a new paper appearing in the journal Nano Letters, Boldizsár Jankó, Pavel Frantsuzov, and Sándor Volkán-Kascó from Notre Dame University reveal a new model, developed to research the blinking phenomena. Their work is preceded by a 2008 paper, which provides a deeper insight into the physical mechanism behind the vast range of on- and off-times in the intermittency. Now, they are confirming that strong correlation exists between the on and off phenomenon.
The experiments conducted by Jankó, Frantsuzov, and Volkán-Kascó were possible thanks to newly developed techniques that did not exist previously. They are suggesting ways to control the blinking process, so scientists could use quantum dots in various fields. Some possible benefits derived from such manipulations are: more stable imaging of cancer cells: real-time images of a viral infection—such as HIV—within a cell, and developing a new generation of brighter display screens for computers, cell phones, and other electronic applications.
Hopefully, future research – based on these groundbreaking experiments – will help materialize the aforementioned benefits.
TFOT has also covered the new quantum sensors developed by researchers from Oxford University, and a research study dealing with superimposed quantum dots that are able to “trap” single electrons.
For more information about the breakthrough on blinking molecules phenomena, see University of Notre Dame’s press release.